Answer :
(a) The rate of reaction is, second order reaction.
(b) The rate of reaction is, zero order reaction.
Explanation :
Rate of reaction : It is defined as the rate of change in concentration of reactant or product with respect to time.
Order of reaction : It is defined as the sum of the exponents or powers to which the molar concentration in the rate law equation are raised to express the observed rate of reaction.
The order of reaction depends on the power of reactant concentration.
(a) The given rate expression is,
![Rate=k[NO_2]^2](https://img.qammunity.org/2020/formulas/chemistry/college/5fuwjfyjdblw1m6xgf08olevz2z5jjsakl.png)
From this expression we conclude that the power of concentration of reactant
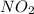 is 2.
is 2.
That means it is a second order reaction.
(b) The given rate expression is,
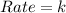
From this expression we conclude that the rate of reaction is equal to rate constant.
That means it is a zero order reaction.