Answer:
molar composition for liquid
xb= 0.24
xt=0.76
molar composition for vapor
yb=0.51
yt=0.49
Step-by-step explanation:
For an ideal solution we can use the Raoult law.
Raoult law: in an ideal liquid solution, the vapor pressure for every component in the solution (partial pressure) is equal to the vapor pressure of every pure component multiple by its molar fraction.
For toluene and benzene would be:
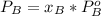
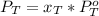
Where:
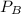 is partial pressure for benzene in the liquid
is partial pressure for benzene in the liquid
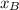 is benzene molar fraction in the liquid
is benzene molar fraction in the liquid
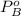 vapor pressure for pure benzene.
vapor pressure for pure benzene.
The total pressure in the solution is:
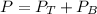
And
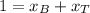
Working on the equation for total pressure we have:
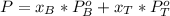
Since
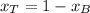
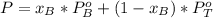
We know P and both vapor pressures so we can clear
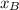 from the equation.
from the equation.
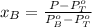
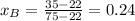
So
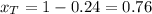
To get the mole fraction for the vapor we know that in the equilibrium:
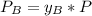
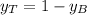
So
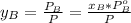
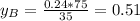
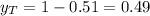
Something that we can see in these compositions is that the liquid is richer in the less volatile compound (toluene) and the vapor in the more volatile compound (benzene). If we take away this vapor from the solution, the solution is going to reach a new state of equilibrium, where more vapor will be produced. This vapor will have a higher molar fraction of the more volatile compound. If we do this a lot of times, we can get a vapor that is almost pure in the more volatile compound. This is principle used in the fractional distillation.