Answer:
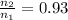
Step-by-step explanation:
Hello, in this case the the volume at both the first and second states are missing, therefore, one could suppose a typical value around 4000 m³ for the first state and 4200 m³ based on their normal operating conditions. In such a way, by considering the normal gas law given in terms of the change of temperature, moles and volume, assuming constant pressure as said on the statement as follows:
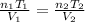
One solves for the increasing mole ratio as shown below:

Thus, the volume at the second state is less than that of the first state.
Best regards.