Answer:
4.18 g
Step-by-step explanation:
The formula for the calculation of moles is shown below:

Given: For Li
Given mass = 2.50 g
Molar mass of Li = 6.94 g/mol
Moles of Li = 2.50 g / 6.94 g/mol = 0.3602 moles
Given: For

Given mass = 2.50 g
Molar mass of
 = 28.02 g/mol
= 28.02 g/mol
Moles of
 = 2.50 g / 28.02 g/mol = 0.08924 moles
= 2.50 g / 28.02 g/mol = 0.08924 moles
According to the given reaction:
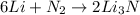
6 moles of Li react with 1 mole of

1 mole of Li react with 1/6 mole of

0.3602 mole of Li react with
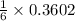 mole of
mole of

Moles of
 that will react = 0.06 moles
that will react = 0.06 moles
Available moles of
 = 0.08924 moles
= 0.08924 moles
 is in large excess. (0.08924 > 0.06)
is in large excess. (0.08924 > 0.06)
Limiting reagent is the one which is present in small amount. Thus,
Li is limiting reagent.
The formation of the product is governed by the limiting reagent. So,
6 moles of Li gives 2 mole of
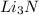
1 mole of Li gives 2/6 mole of
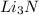
0.3602 mole of Li react with
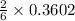 mole of
mole of
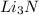
Moles of
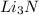 = 0.12
= 0.12
Molar mass of
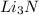 = 34.83 g/mol
= 34.83 g/mol
Mass of
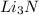 = Moles × Molar mass = 0.12 × 34.83 g = 4.18 g
= Moles × Molar mass = 0.12 × 34.83 g = 4.18 g
Theoretical yield = 4.18 g