Answer:
The concentration solution by percent in mass is 8.28%
Step-by-step explanation:
Molarity is moles of solute present in 1L of its solution .
Hence the formula is
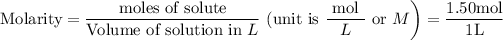
Let us convert the numerator and the denominator into mass in g
Numerator :
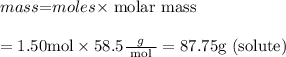
Denominator :
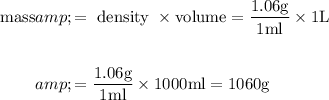
The formula to find mass percentage is
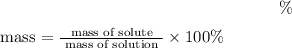
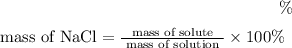
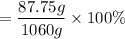
=8.28% (Answer)