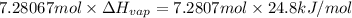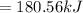Answer:
180.56 Kilo joules of energy is removed in the form of heat when 1.00 kg of freon-11 is evaporated.
Step-by-step explanation:
Molar mass of freon-11 = 137.35 g/mol
Enthalpy of vaporization of freon-11=
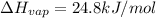
Mass of freon-11 evaporated = 1.00 kg = 1000 g
Moles of freon-11 evaporated =

Energy in the form of heat removed when 1.00 kg of freon-11 gets evaporated: