Answer:
Mass of sodium + nitrogen +oxygen+ oxygen+ oxygen = =85 g/mol
Step-by-step explanation:
Molar mass is the mass of 1 mole of the substance and its unit is g/mol
We find the molar mass by just adding atomic mass of the elements present in the chemical formula of the compound
For example
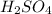
Atomic mass of H is 1.008 (approximately 1)
Atomic mass of S is 32
Atomic mass of O is 16
There are 2, H atoms 1, S atom and 4, O atoms
So, the molar mass of the compound
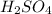 will be
will be
![\\$=[(2 * 1)+(1 * 32)+(4 * 16)]$\\\\$=(2+32+64)=98 (g)/(m o l)$](https://img.qammunity.org/2020/formulas/chemistry/middle-school/k3tk6xdfga678yl40zc820z85x1cqxkcy6.png)
The question says 1, Na
1, N and
3, O
So the chemical formula we get is
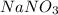
The molar mass of the compound
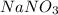 is
is
![\\$=[(1 * 23)+(1 * 14)+(3 * 16)]$\\\\$=(23+14+48)$](https://img.qammunity.org/2020/formulas/chemistry/middle-school/bmhbc0tqynxpnl251o8540z0az4jj6jnuu.png)
=85 g/mol (Answer)