Answer:
ΔT=-747,13°C
Step-by-step explanation:
Sensible heat is the amount of thermal energy that is required to change the temperature of an object, the equation for calculating the heat change is given by:
Q=msΔT
where:
- Q, heat that has been absorbed or realeased by the substance [J]
- m, mass of the substance [g]
- s, specific heat capacity [J/g°C] (
- ΔT, changes in the substance temperature [°C]
To solve the problem, we clear ΔT of the equation and then replace our data:
Q=msΔT
ΔT=Q/ms
Δ
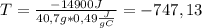 °C
°C
(Note that Q=-14900 J because there is a LOST of thermal energy)
Thus, the change in temperature of the steel bar is -747,13°C, meaning that the temperature of the bar decreases.