Answer:
Specific rotation = -258°
Optical purity = 200%
Step-by-step explanation:
Substances that have a chiral carbon ( a carbon which has four different atoms or compounds bond to it) have optical isomers. The different molecules are called enantiomers, and they differ by the side they deflect polarised light. A mixture contained the same number of the enantiomers is not optical and doesn't deflect polarized light. It's called a racemic mixture.
The specific rotation (spec.rot.) can be calculated by the equation:
spec.rot. = α/lc
Where α is the observed rotation of the plane polarised light in degrees, l is the path length in decimeters, and c is the concentration of the solution in g/100 mL.
The initial concentration is 10g/500mL = 2g/100 mL. The racemic mixture doesn't change the specific rotation, so the final concentration is 10g/1000 mL = 1g/100 mL. Then:
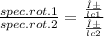
with α and l the same:
spec.rot.1/spec.rot.2 = c2/c1
spec.rot.2 = c1xspec.rot.1/c2
spec.rot.2 = 2x(-129°)/1
spec.rot.2 = -258°
If for a -129° the optical purity was 100%:
-129° ---------- 100%
-258° --------- x
By a direct simple three rule:
129x = 25800
x = 200%