Answer : The section of the periodic table contains this element is, (light blue color) section.
Explanation :
An alkali metal : These are the elements which lie in group 1.
Their general electronic configuration is:
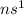 where n is the outermost shell.
where n is the outermost shell.
An alkaline earth metal : These are the elements which lie in group 2.
Their general electronic configuration is:
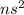 where n is the outermost shell.
where n is the outermost shell.
Non-metals : They are the elements that are present in groups 14 to 17 of the periodic table.
Their general electronic configuration is:
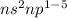 where n is the outermost shell.
where n is the outermost shell.
Transition elements : They are the elements which lie between 's' and 'p' block elements. These are the elements which lie in group 3 to 12. The valence electrons of these elements enter d-orbital.
Their general electronic configuration is: where n is the outermost shell.
In the periodic table, the metals are present on the left side (light green color), non-metals are present on the right side (light blue color), the transition elements are present in between metal an non-metal (light pink color) and actinoids and lanthanoids are present at below periodic table (light yellow color).
From this we conclude that, non-metal (light blue color) atoms have a partially filled p-sublevel.
Hence, the section of the periodic table contains this element is, (light blue color) section.