Answer:
pCH₄ = 105.1 - 0.42 = 104.68 torr
pI₂ = 7.96 -0.42 = 7.54 torr
pCH₃I = 0.42 torr
pHI = 0.42 torr
Step-by-step explanation:
Kp is the equilibrium constant for the partial pressure of the gases in the reaction, and it is calculated for a general equation:
aA(g) + bB(g) ⇄ cC(g) + dD(g)
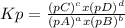 , where p is the partial pressure in the equilibrium. By the reaction given:
, where p is the partial pressure in the equilibrium. By the reaction given:
CH₄(g) + I₂(g) ⇄ CH₃I(g) + HI(g)
105.1 torr 7.96 torr 0 0 initial partial pressure
-x -x +x +x react
105.1-x 7.96-x x x equilibrium
Then:
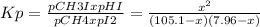
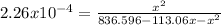
x² = 0.1891 - 0.0255x -2.26x10⁻⁴x²
0.9997x² + 0.0255x - 0.1891 = 0
Using Bhaskara's rule:
Δ = (0.0255)² - 4x(0.9997)x(-0.1891)
Δ = 0.7568
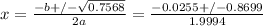
Using only the positive term, x = 0.42 torr.
So,
pCH₄ = 105.1 - 0.42 = 104.68 torr
pI₂ = 7.96 -0.42 = 7.54 torr
pCH₃I = 0.42 torr
pHI = 0.42 torr