Answer:
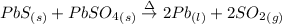
Step-by-step explanation:
The balanced reaction of heating solid lead (II) sulfide with the solid lead (II) sulfate to produce liquid lead and sulfur dioxide gas is shown below:
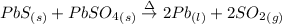
In the balance reaction above, all the phases are indicated. This reaction is used for the production of lead metal.