Step-by-step explanation:
The given data is as follows.
Change in Potential Energy =
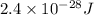
Formula for change in potential energy is as follows
Change in Potential Energy =
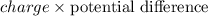
Hence, the potential difference between the two points will be as follows.
V =
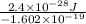
=
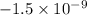 volt
volt
Therefore, the potential due to two charge initially is as follows.
V =
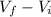
=
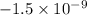 volt
volt
Hence,
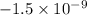 volt =
volt =
![9 * 10^(9) * 1.602 * 10^(-19)[(1)/(r) - (1)/(0.52)]](https://img.qammunity.org/2020/formulas/chemistry/college/sfo6as6fx0hf6kd0sbmfnhyi90k06rng8t.png)
r = 0.493 m = 49.3 cm (as 1 m = 100 cm)
Thus, we can conclude that the final distance between the electron and proton is 49.3 cm.