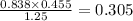Answer:
V1= 0.305L
Step-by-step explanation:
To find the initial volume of 1.25M potassium fluoride needed to make tge dilution specified in the question, we can use: C1 × V1 = C2 × V2
Since the question wants the volume in litres, convert 455 mL to L
455/ 1000
= 0.455 L
Now make the substitution
1.25 × V1 = 0.838 × 0.455
Rearrange to make V1 the subject
V1=