Answer:
The weak base B) does not dissociate completely.
Step-by-step explanation:
Bases are the substance which produces
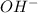 ions when dissolved in aqueous solution or when they are treated with water. The pH of bases remains above 7. They react with salt and cause neutralization a process through which salts are formed.
ions when dissolved in aqueous solution or when they are treated with water. The pH of bases remains above 7. They react with salt and cause neutralization a process through which salts are formed.
Based upon the dissociation rate, they are classified as strong base or week base. The base which gets completely dissolved in aqueous solution or water, it is called as strong base, whereas the base which gets partially dissociates or does not dissociate completely when treated with water or aqueous solution are called weak base.