Answer:
The weak acid D) acetic acid.
Step-by-step explanation:
Acid are the substance which has pH below 7 and releases
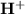 ions when they are treated with aqueous solution. Based upon the rate of dissociation, the acids are classified as strong acids which has high dissociation rate and weak acid with low dissociation rate.
ions when they are treated with aqueous solution. Based upon the rate of dissociation, the acids are classified as strong acids which has high dissociation rate and weak acid with low dissociation rate.
Acetic acid is a organic acid with the formula
 . It is a colorless acid and also named as ethonoic acid.
. It is a colorless acid and also named as ethonoic acid.
Among the given list of acid in the option which includes, Sulfuric acid, Nitric acid, Hydrochloric acid, and Acetic acid, first three are strong acids, whereas the lastly mentioned is a weaker one.