Answer:
"How does the volume of a gas kept at constant pressure change as its temperature is increased?"
Step-by-step explanation:
One possible question can be:
"How does the volume of a gas kept at constant pressure change as its temperature is increased?"
The answer to this question is contained in Charle's law, which states that for a gas at constant pressure, the volume of the gas is proportional to its absolute temperature:
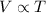
Or also written as
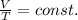
By looking at this equation, we can find immediately the answer to our question: as the (absolute) temperature of the gas increases, the volume increases as well, by the same proportion.