Answer:
The statement is False.
Step-by-step explanation:
A mixture contains two or more elements present in it.
The elements are not in chemical combination with each other.
A compound too contains two or more elements but they are in chemical combination.
For example NaCl is a compound which contains 1 part of Sodium and 1 part of Chlorine.
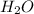 is a compound 2g of hydrogen is chemically combined with 16g of Oxygen.
is a compound 2g of hydrogen is chemically combined with 16g of Oxygen.
Chemical bonding is the attractive forces which holds the atoms together in a molecule.
NaCl - the attractive force is called as Ionic bond.
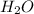 - the bond present is a covalent bond
- the bond present is a covalent bond
A mixture has two or more elements but there is no chemical bonding present in it. The components of a mixture can be easily separated from it.