Answer:
a. 0.528 M ..
Step-by-step explanation:
Hello!
In this case, since the given by-mass percent can be written as:
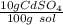
By using the density and molar mass of the solute, cadmium sulfate, we can compute the molarity, by also making sure we convert from mL to L of solution:
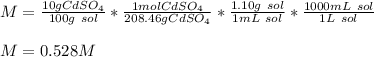
Thereby, the answer is a. 0.528 M .
Best regards.