Answer:
31 moles
Step-by-step explanation:
The balanced combustion reaction of the wax,
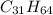 is shown below as:
is shown below as:
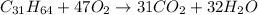
As seen from the reaction,
1 mole of wax,
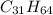 on combustion produces 31 moles of carbon dioxide,
on combustion produces 31 moles of carbon dioxide,

Hence, moles of
 when 1 mole of wax,
when 1 mole of wax,
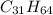 is burnt = 31 moles
is burnt = 31 moles