Answer:
Sulfur dioxide is a covalent bond because we have two non-metals (Sulfur and Oxygen).
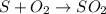 (Sulfur dioxide formula)
(Sulfur dioxide formula)
It's called Sulfur dioxide but it can be called Sulfurous anhydride and Sulfur (lV) oxide.
Sulfur dioxide can have different reactions:
- In the presence of oxygen oxidation of sulfur dioxide to sulfur trioxide occurs and the formula is:
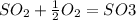
The equal is because it's a reversible equation. This reaction is spontaneous.
- The sulfur trioxide reacting with water produces sulfuric acid. The formula is:
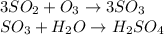
- Also the sulfur dioxide reacting with sodium hydroxide produces sodium sulfite and the formula is:
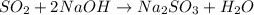
- And finally we have the reduction of sulfur dioxide (it can be in presence of hydrogen sulfide) getting elemental sulfur and water. The formula is:
