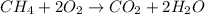Answer: Option (a) is the correct answer.
Step-by-step explanation:
The given reaction is as follows.
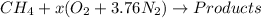
Since, it is a combustion reaction so, nitrogen will not take part in it and hence, it will remain the same on both sides of the reaction.
Also, it is known that in a combustion reaction oxygen reacts with a hydrocarbon and results in the formation of carbon dioxide and water. Therefore, for the above reaction we write the complete reaction equation as follows.
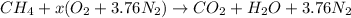
or,
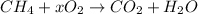 as nitrogen is not taking part in the reaction.
as nitrogen is not taking part in the reaction.
Number of atoms on reactant side are as follows.
C = 1
H = 4
O = 2
Number of atoms on product side are as follows.
C = 1
O = 3
H = 2
Therefore, to balance this equation we multiply oxygen on reactant side by 2. Also, we multiply water on product side by 2. Hence, the complete balanced chemical equation is as follows.