Answer:
a)CN=12
b)APF=74 %
c)a=0.35 nm
d)ρ=9090.9
Step-by-step explanation:
Given that
Nickel have FCC structure
We know that in FCC structure ,in FCC 8 atoms at corner with 1/8 th part in one unit cell and 6 atoms at faces with 1/2 part in one unit cell .
Z=8 x 1/8 + 1/2 x 6 =4
Z=4
Coordination number (CN)
The number of atoms which touch the second atoms is known as coordination number.In other word the number of nearest atoms.
CN=12
Coordination number of FCC structure is 12.These 12 atoms are 4 atoms at the at corner ,4 atoms at 4 faces and 4 atoms of next unit cell.
APF
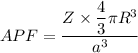
We know that for FCC
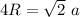
Now by putting the values
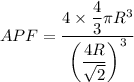
APF=0.74
APF=74 %
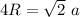
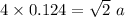
a=0.35 nm
Density
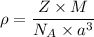
We know that M for Ni
M=58.69 g/mol
a=0.35 nm
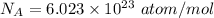
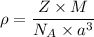
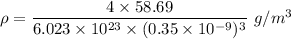
ρ=9090.9