Answer : The energy required is, 574.2055 KJ
Solution :
The conversions involved in this process are :
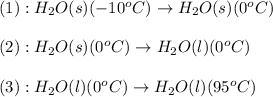
Now we have to calculate the enthalpy change or energy.
![\Delta H=[m* c_(p,s)* (T_(final)-T_(initial))]+n* \Delta H_(fusion)+[m* c_(p,l)* (T_(final)-T_(initial))]](https://img.qammunity.org/2020/formulas/chemistry/college/c9df2bwyod8bzndytaseet5mjtidhivx9q.png)
where,
 = energy required = ?
= energy required = ?
m = mass of ice = 1 kg = 1000 g
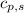 = specific heat of solid water =
= specific heat of solid water =
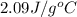
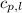 = specific heat of liquid water =
= specific heat of liquid water =
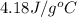
n = number of moles of ice =
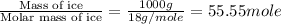
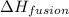 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
Now put all the given values in the above expression, we get
![\Delta H=[1000g* 4.18J/gK* (0-(-10))^oC]+55.55mole* 6010J/mole+[1000g* 2.09J/gK* (95-0)^oC]](https://img.qammunity.org/2020/formulas/chemistry/college/9wnqc4bipyerceo0o3zt9xxivqyys58htq.png)
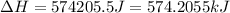 (1 KJ = 1000 J)
(1 KJ = 1000 J)
Therefore, the energy required is, 574.2055 KJ