Step-by-step explanation:
Formula for compressibility factor is as follows.
z =
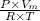
where, z = compressibility factor for helium = 1.0005
P = pressure
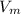 = molar volume
= molar volume
R = gas constant = 8.31 J/mol.K
T = temperature
So, calculate the molar volume as follows.

=

= 0.0056
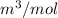
As molar mass of helium is 4 g/mol. Hence, calculate specific volume of helium as follows.

=
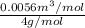
= 0.00139
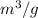
= 0.00139
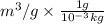
= 1.39
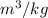
Thus, we can conclude that the specific volume of Helium in given conditions is 1.39
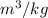 .
.