Answer: 1. The molecular weight of the compound is 222.8 g/mol
2. The probable molecular formula of the solute is
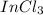
Step-by-step explanation:
Elevation in boiling point is given by:
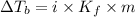
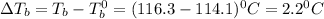 = elevation in boiling point
= elevation in boiling point
i= vant hoff factor = 1 (for non electrolyte)
 = boiling point constant =
= boiling point constant =
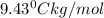
m= molality
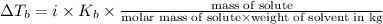
Weight of solvent (tin chloride)= 50.0 g =0.05 kg
Molar mass of unknown solute = M g/mol
Mass of unknown solute = 2.6 g
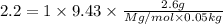

The possible formula for the compound would be
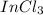 as indium has valency of 3 and chlorine has valency of 1 has molecular mass almost equal to 222.8.
as indium has valency of 3 and chlorine has valency of 1 has molecular mass almost equal to 222.8.