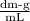Answer:
[α] = -77.5° /
Step-by-step explanation:
Given;
Mass of optically pure substance in the solution = 10 g
Volume of water = 500 mL
Length of the polarimeter, l = 20 cm = 20 × 0.1 dm = 2 dm
measured rotation = - 3.10°
Now,
The specific rotation ( [α] ) is given as:
[α] =
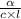
here,
α is the measured rotation = -3.10°
c is the concentration
or
c =
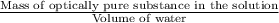
or
c =
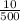
or
c = 0.02 g/mL
on substituting the values, we get
[α] =
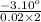
or
[α] = -77.5° /