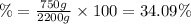Answer:
750 grams is the mass of product and 34.09% is the compositions of the product.
Step-by-step explanation:
Container 1 , 30% of propane by mass:
Container 2 , 60% of propane by mass:
Mass of mixture in container-1 = 1000 g
Mass of propane in 1000 g of mixture = m
m=
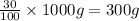
Mass of mixture in container-2 = 1200 g
Mass of propane in 1200 g of mixture = m'
m'=
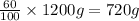
After mixing 1000 grams and 1200 grams of mixture
Final mass of mixtures = 1000 g + 1200 g = 2200 g
Final mass of propane = 300 g+ 720 g = 750 g
Composition of propane after mixing of mixtures :