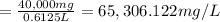Answer:
The concentration of SiC chips is 65,306.122 mg/L.
Step-by-step explanation:
Mass of silicon carbide ,m= 40 g
Density of silicon =
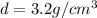
Volume of silicon carbide = v

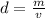
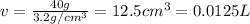
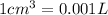
Volume of toluene = V = 600 mL = 0.6 L
Volume of solution = v + V = 0.0125 L + 0.6 L =0.6125 L
The concentration of SiC chips in mg/L:
m = 40 g = 40,000 mg
(1 g = 1000 mg)