Answer:
Electron
Step-by-step explanation:
See attached figure (it was missing in the question).
An atom consists of three types of particle:
- Proton: it is positively charged (charge
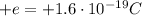 ) and it is located in the nucleus. Its mass is approximately
) and it is located in the nucleus. Its mass is approximately
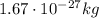
- Neutron: it has no electric charge, and it is also located in the nucleus. Its mass is just slighly larger than that of the proton (but it is also approximated to
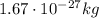 )
)
- Electron: it is negatively charged (charge
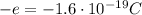 ) and it orbits around the nucleus. Its mass is much smaller than that of the proton (
) and it orbits around the nucleus. Its mass is much smaller than that of the proton (
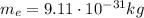 )
)
From the figure, we see that the only particle orbiting around the nucleus is particle A: therefore, A must be an electron.