Answer:
The unit of A must be 'atm', and the units of B and C must be 'atmxK'.
If a system is at steady state it means the properties do not vary over time.
Step-by-step explanation:
The units of pressure must be atm, bar, Pa or mmHg. If we use the atm unit the result of the equation should be the pressure in 'atm'. Thus, the unit of A must be 'atm', and the units of B and C must be 'atmxK'. So, If we replace the equation with the temperatures (T) in Kelvin (K) the result will be in 'atm'.
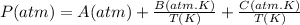
Problem 2: If a system is at steady state it means the properties do not vary over time. This is the definition of a steady state. Also, every particular steady state will define their own properties but each steady state will no vary their properties over time.