Step-by-step explanation:
Molar mass of
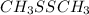 is 94 g/mol. As it is known that number of moles is equal to mass of a substance divided by its molar mass.
is 94 g/mol. As it is known that number of moles is equal to mass of a substance divided by its molar mass.
Then, calculate the number of moles as follows.
No. of moles =
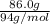 in 1 s
in 1 s
= 0.914 mol
So, in 60 sec number of moles will be equal to 0.914 x 60 = 54.89 mol/min.
Hence, the molar flow rate = 54.89 mol/min
Also, density is equal to mass of a substance divided by its volume.
Density =

Volume =
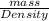
=
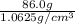
= 80.941

As, 80.941
 of volume flows in 1 s . Therefore, flow of volume in 1 hour will be calculated as follows.
of volume flows in 1 s . Therefore, flow of volume in 1 hour will be calculated as follows.
In 1 hr = 80.941

= 291388.24
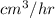
Since, 1
 = 0.001 L.
= 0.001 L.
So, 291388.24
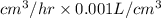
= 291.38824 L/hr
Thus, we can conclude that molar flow rate in mol/min is 54.89 mol/min and the volumetric flow rate in L/hr is 291.38824 L/hr.