Step-by-step explanation:
The given data is as follows.
Mass flow rate of mixture = 1368 kg/hr
 in feed = 40 mole%
in feed = 40 mole%
This means that
 in feed = (100 - 40)% = 60%
in feed = (100 - 40)% = 60%
We assume that there are 100 total moles/hr of gas
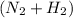 in feed stream.
in feed stream.
Hence, calculate the total mass flow rate as follows.
40 moles/hr of N_{2}/hr (28 g/mol of
 ) + 60 moles/hr of
) + 60 moles/hr of
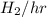 (2 g/mol of
(2 g/mol of
 )
)
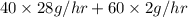
= 1120 g/hr + 120 g/hr
= 1240 g/hr
=
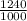 (as 1 kg = 1000 g)
(as 1 kg = 1000 g)
= 1.240 kg/hr
Now, we will calculate mol/hr in the actual feed stream as follows.
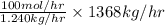
= 110322.58 moles/hr
It is given that amount of nitrogen present in the feed stream is 40%. Hence, calculate the flow of
 into the reactor as follows.
into the reactor as follows.
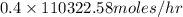
= 44129.03 mol/hr
As 1 mole of nitrogen has 28 g/mol of mass or 0.028 kg.
Therefore, calculate the rate flow of
 into the reactor as follows.
into the reactor as follows.
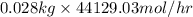
= 1235.612 kg/hr
Thus, we can conclude that the the feed rate of pure nitrogen to the mixer is 1235.612 kg/hr.