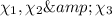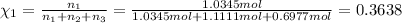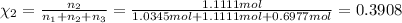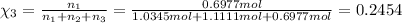Answer:
The mole fraction composition of the liquid is :
Mole fraction of butane, pentane and hexane are 0.3638,0.3908 and 0.2454 respectively.
Step-by-step explanation:
Mass of the liquid mixture = 200 g
Percentage of butane = 30%
Mass of butane =
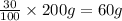
Moles of butane =
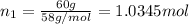
Percentage of pentane= 40%
Mass of pentane=
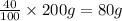
Moles of pentane=
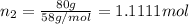
Percentage of hexane = 100% - 30% - 40% = 30%
Mass of hexane =
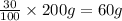
Moles of hexane =
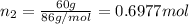
Mole fraction of butane, pentane and hexane :