Step-by-step explanation:
(a) Mass given = 225 g
Molecular mass of
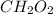 =
=
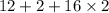 = 12 + 2 + 32 = 46 g/mol
= 12 + 2 + 32 = 46 g/mol
As, it is known that number of moles is equal to the mass divided by molar mass.
Mathematically, No. of moles =
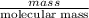
Hence, calculate the number of moles of formic acid as follows.
No. of moles =
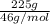
= 4.89 mol or g mol
Hence, in 225 g of the compound there are 4.89 mol or g mol.
(b) It is known that 1 g = 0.0022 pound
So, pounds present in 4.89 g will be calculated as follows.
4.89 \times 0.0022 pound
= 0.0107 pound mole
Hence, in 225 g of the compound there are 0.0107 pound mole.