Answer: 0.102 Liters
Step-by-step explanation
According to the ideal gas equation:

P = Pressure of the gas =
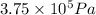 = 3675 atm (1 kPa= 0.0098 atm)
= 3675 atm (1 kPa= 0.0098 atm)
V= Volume of the gas = ?
T= Temperature of the gas = 23.6°C = 296.6 K
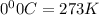
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas = 45.6
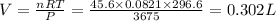
Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
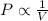 (At constant temperature and number of moles)
(At constant temperature and number of moles)
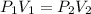
where,
 = initial pressure of gas =
= initial pressure of gas =
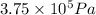
 = final pressure of gas =
= final pressure of gas =
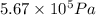
 = initial volume of gas = 0.302 L
= initial volume of gas = 0.302 L
 = final volume of gas = ?
= final volume of gas = ?
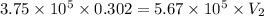
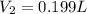
The final volume has to be 0.199 L, thus (0.302-0.199) L= 0.102 L must release into the atmosphere.
Therefore the answer is 0.102 L