Answer : The correct option is, (b) 0.087
Explanation :
The formula used for relative saturation is:
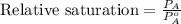
where,
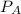 = partial pressure of ethyl acetate
= partial pressure of ethyl acetate
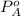 = vapor pressure of ethyl acetate
= vapor pressure of ethyl acetate
Given:
Relative saturation = 50 % = 0.5
Vapor pressure of ethyl acetate = 16 kPa
Now put all the given values in the above formula, we get:
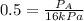
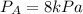
Now we have to calculate the molar saturation.
The formula used for molar saturation is:
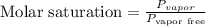
and,
P(vapor free) = Total pressure - Vapor pressure
P(vapor) =
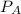 = 8 kPa
= 8 kPa
So,
P(vapor free) = 100 kPa - 8 kPa = 92 kPa
The molar saturation will be:
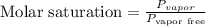
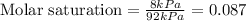
Therefore, the molar saturation is 0.087