Answer:
Complete reaction
mole %: 0% N₂; 20% H₂; 80% NH₃
Gram %: 0% N₂; 2,9% H₂; 97,1% NH₃
75% reaction:
mole%: 8,3% N₂; 41,7% H₂; 50% NH₃
Gram: 20% N₂; 7,2% H₂; 72,8% NH₃
Step-by-step explanation:
The global reaction in the reactor is:
N₂(g) + 3 H₂ (g) → 2 NH₃ (g)
For a complete reaction of 20 moles of N₂(g) you need:
20 moles N₂ ₓ
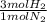 = 60 moles of H₂(g)
= 60 moles of H₂(g)
So, 10 moles of H₂(g) will stay in the end.
The moles produced of NH₃ are:
20 moles N₂ ₓ
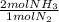 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)
Thus, the final composition in moles is:
0 moles N₂; 10 moles H₂; 40 moles of NH₃
The mole % is:
0% N₂; ¹⁰/₅₀ ₓ 100 = 20% H₂; ⁴⁰/₅₀ ₓ100 = 80% NH₃
In grams you have:
10 moles H₂
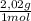 = 20,2 g of H₂
= 20,2 g of H₂
40 moles NH₃
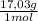 = 681,2 g of NH₃
= 681,2 g of NH₃
0% N₂;
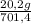 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂;
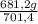 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃
With a 75% of conversion you have that the moles produced of NH₃ are:
40 moles of NH₃(g) × 75% = 30 moles of NH₃
The necessary moles to produce these moles of NH₃ are:
30 moles NH₃ ₓ
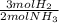 = 45 moles of H₂(g)
= 45 moles of H₂(g)
So, 25 moles of H₂(g) will stay in the end.
30 moles NH₃ ₓ
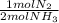 = 15 moles of N₂(g)
= 15 moles of N₂(g)
So, 5 moles of H₂(g) will stay in the end.
Thus, the final composition in moles is:
5 moles N₂; 25 moles H₂; 30 moles of NH₃
The mole % is:
⁵/₆₀ ₓ 100 =8,3% N₂; ²⁵/₆₀ ₓ 100 = 41,7% H₂; ³⁰/₆₀ ₓ100 = 50% NH₃
In grams you have:
5 moles N₂
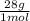 = 140 g of N₂
= 140 g of N₂
25 moles H₂
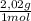 = 50,5 g of H₂
= 50,5 g of H₂
30 moles NH₃
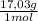 = 510,9 g of NH₃
= 510,9 g of NH₃
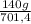 ₓ 100 =20% N₂;
ₓ 100 =20% N₂;
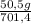 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂;
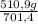 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃
I hope it helps!