Answer:
Degree of ionization = 0.0377
pH of the solution = 1.769
Step-by-step explanation:
Initial concentration of HF = 0.45 M
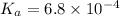
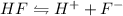
Initial 0.45 0 0
At equi 0.45 - x x x
Equilibrium constant =
![([H^+][F^-])/(HF)](https://img.qammunity.org/2020/formulas/chemistry/college/y75zxqh1jzq2m5hfcikkkbh1ludx02g8ou.png)
![6.8 * 10^(-4)= ([x][x])/(0.45 - x)](https://img.qammunity.org/2020/formulas/chemistry/college/emi2wtfdm7n42ib30m2h9o8olez7m28r23.png)
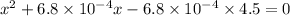
x = 0.017 M
x = Cα
α = Degree of ionization
C = Concentration
Degree of ionization =
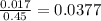
![pH = -log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/college/gny7xuakxqc45obx08p22znzokdd3vdn81.png)
[H^+]=0.017 M
![pH = -log[0.017]](https://img.qammunity.org/2020/formulas/chemistry/college/ovoq4znubay0ypiar6yp3vvcickmh8gfzn.png)
= 1.769