Answer:
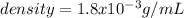
Step-by-step explanation:
Hello,
Considering the ideal equation of state:

The moles are defined in terms of mass as follows:
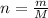
Whereas
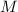 the gas' molar mass, thus:
the gas' molar mass, thus:
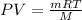
Now, since the density is defined as the quotient between the mass and the volume, we get:
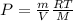
Solving for
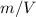 :
:
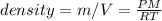
Thus, the result is given by:
![density=((1atm)(44g/mol))/([0.082atm*L/(mol*K)]*298.15K) \\density=1.8g/L=1.8x10^(-3)g/mL](https://img.qammunity.org/2020/formulas/chemistry/college/ujfz7djt3gevc4d9ebm88vcnjvgxfakq6m.png)
Best regards.