Answer: The correct answer is Option C.
Step-by-step explanation:
The chemical formula for trinitrotoluene is
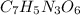
In 1 mole of TNT, 7 moles of carbon atom, 5 moles of hydrogen atom, 3 moles of nitrogen atom and 6 moles of oxygen atom are present.
We know that:
Mass of trinitrotoluene = 227.1 g/mol
Mass of carbon = 12.01 g/mol
We are given:
Mass of TNT = 57.6 grams
To calculate the mass of carbon in given amount of TNT, we apply unitary method:
In 227.1 grams of TNT, amount of carbon present is =
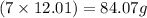
So, in 57.6 grams of TNT, the amount of carbon present is =
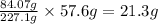
Hence, the correct answer is Option C.