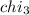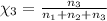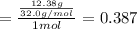Answer:
Mole fraction of ethanol is 0.363.
Mole fraction of methanol is 0.387.
Step-by-step explanation:
Mole fraction of water =
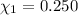
Mole fraction of ethanol =
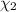
Mole fraction of methanol =
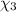
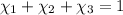
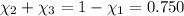
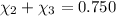
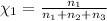
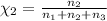
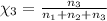
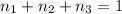
Moles of water =
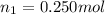
Moles of ethanol=
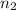
Moles of methanol=
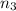
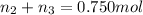
Mass of the mixture = M
Mass of water,
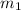
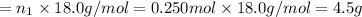
Fraction of water by mass = 0.134
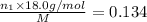
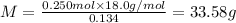
Mass of ethanol =
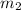
Mass of methanol =
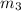
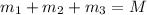
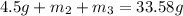
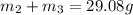 ..[1]
..[1]
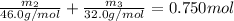
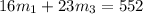 ..[2]
..[2]
On solving [1] and [2]:
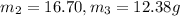
Mole fraction of ethanol =
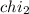
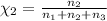
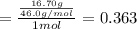
Mole fraction of methanol =