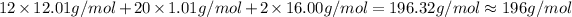Answer:
1) The density of air at 40 degrees Celsius and 3 atm pressure is 3.4 g/L.
2) Molecular mass of linanyl acetate is 196 g/mol.
Step-by-step explanation:
1) Average molecular weight of an air ,M= 28.97 g/mol

or
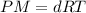
P = Pressure of the gas
T = Temperature of the gas
d = Density of the gas
M = molar mass of the gas
R = universal gas constant
P = 3 atm, T = 40°C = 313.15 K, M = 28.97 g/mol
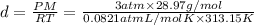
d = 3.4 g/mL
The density of air at 40 degrees Celsius and 3 atm pressure is 3.4 g/L.
2) Molecular formula of Linanyl acetate =
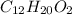
Atomic mass sof carbon = 12.01 g/mol
Atomic mass of hydrogen = 1.01 g/mol
Atomic mass of oxygen = 16.00 g/mol
Molecular mass of Linanyl acetate :