Answer:
22.22 grams of an alloy is present as liquid and 77.78 g is present as solid at given temperature.
Step-by-step explanation:
Let the mass of alloy in liquid be x and mass of alloy in solid be y.
Total mass of the alloy = 100 g
x + y = 100 g..[1]
Percentage of component B in alloy = 60%
Total Mass of component B in an alloy =
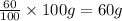
25 % of the liquid is component B
Mass of component B in liquid = a
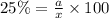
a = 0.25 x
70% of solid is component B.
Mass of component B in liquid = b
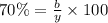
b = 0.7 y
a + b = 60
0.25 x+ 0.7 y= 60 g...[2]
On solving equation [1] and [2] , we get:
x = 22.22 g
y = 77.78 g