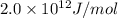Answer : The nuclear binding energy of He for one mole is
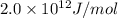
Explanation :
The isotopic representation of He :
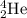
Atomic number = Number of protons = 2
Mass number = 4
Number of neutrons = Mass number - Atomic number = 4 - 2 = 2
To calculate the mass defect of the nucleus, we use the equation:
![\Delta m=[(n_p* m_p)+(n_n* m_n)+]-M](https://img.qammunity.org/2020/formulas/chemistry/college/4qqy8kpy0j3mnfwfjymm21jcgu6c406eet.png)
where,
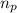 = number of protons = 2
= number of protons = 2
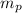 = mass of one proton = 1.00728 amu
= mass of one proton = 1.00728 amu
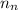 = number of neutrons = 2
= number of neutrons = 2
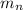 = mass of one neutron = 1.00866 amu
= mass of one neutron = 1.00866 amu
M = Nuclear mass number = 4.00150 amu
Putting values in above equation, we get:
![\Delta m=[(2* 1.00728)+(2* 1.00866)]-[4.00150]\\\\\Delta m=0.03038amu](https://img.qammunity.org/2020/formulas/chemistry/college/m7swtwwbznjepgr32jejfp1ozcwraeu6az.png)
Now converting the value of amu into kilograms, we use the conversion factor:
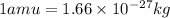
So,
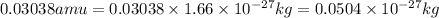
To calculate the equivalent energy, we use the equation:

E = nuclear binding energy = ?
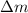 = mass change =
= mass change =
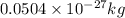
c = speed of light =
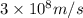
Putting values in above equation, we get:
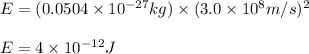
Nuclear binding energy for one mole is:
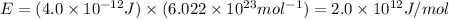
Therefore, the nuclear binding energy of He for one mole is