Answer:
4714.950 kilograms is the mass of a sample containing 45.0 kmol of methyl acetate.
Step-by-step explanation:
Moles of methyl acetate =
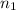 =45.0 kmol= 45000 mol
=45.0 kmol= 45000 mol
Mole percentage of methyl acetate = 60.0%
Total moles in the sample = n
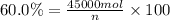
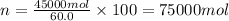
Mole percentage of methyl alcohol = 20.0%
Moles of methyl alcohol = n_2
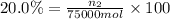

Mass of methyl alcohol =
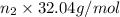
=
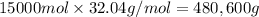
Mole percentage of acetic acid = 20.0%
Moles of acetic acid = n_3
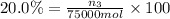
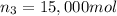
Mass of acetic acid=
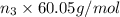
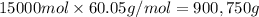
Mass of methyl methyl acetate=
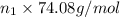

Mass of sample: 480,600 g + 3,333,600 g + 900,750 g = 4714950 g
4714950 g = 4714.950 kg
(1 kg = 1000 g)