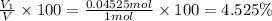Answer:
4.525% is the percentage by volume of oxygen in the gas mixture.
Step-by-step explanation:
Total pressure of the mixture = p = 4.42 atm
Partial pressure of the oxygen =
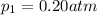
Partial pressure of the helium =
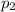
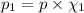 (Dalton law of partial pressure)
(Dalton law of partial pressure)
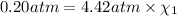
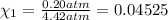
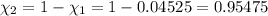
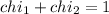
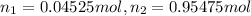
According Avogadro law:
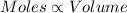 (At temperature and pressure)
(At temperature and pressure)
Volume occupied by oxygen gas =

Total moles of gases = n = 1 mol
Total Volume of the gases = V
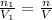
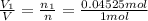
Percent by volume of oxygen in the gas mixture: