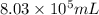Answer: The volume of solution is
Step-by-step explanation:
The relationship between specific gravity and density of a substance is given as:
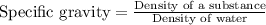
Specific gravity of sulfuric acid solution = 1.22
Density of water = 1.00 g/mL
Putting values in above equation we get:
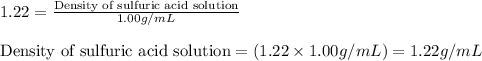
We are given:
25% (m/m) sulfuric acid solution. This means that 25 g of sulfuric acid is present in 100 g of solution
Conversion factor: 1 kg = 1000 g
Mass of solution having 254 kg or 245000 g of sulfuric acid is calculated by using unitary method:
If 25 grams of sulfuric acid is present in 100 g of solution.
So, 245000 grams of sulfuric acid will be present in =
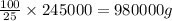
To calculate volume of a substance, we use the equation:
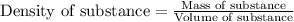
Density of solution = 1.22 g/mL
Mass of Solution = 980000 g
Putting values in above equation, we get:
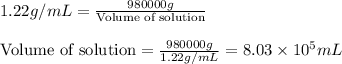
Hence, the volume of solution is