Step-by-step explanation:
1) Edge length of the metal in BCC unit cell =
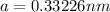
Atomic radius of the metal atom = r
For BCC unit cell, relationship between edge length and radius is given as:
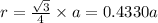
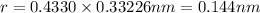
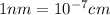
![r=0.144 nm=0.1439* 10^(-7) cm=1.44* 10^(-8) cm[\tex]</p><p><strong>The atomic radius of the metal atom in BCC unit cell is [tex]1.44 * 10^(-8) cm](https://img.qammunity.org/2020/formulas/chemistry/college/9putbeljkgokzbwd97f7mu44o8wf43px9i.png) .
.
2) Edge length of the metal in FCC unit cell =
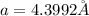
Atomic radius of the metal atom = r
For FCC unit cell, relationship between edge length and radius is given as:
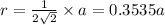
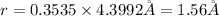
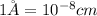
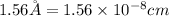
The atomic radius of the metal atom in FCC unit cell is
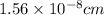 .
.