Answer : The volume of
 gas is 1.00 L
gas is 1.00 L
Explanation :
First we have to determine the moles of
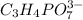 .
.
Molar mass of
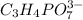 = 182.9 g/mole
= 182.9 g/mole
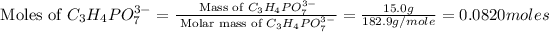
Now we have to calculate the moles of
 .
.
The given balanced chemical reaction is:
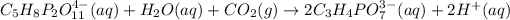
From the reaction we conclude that,
As, 2 moles of
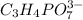 produce from 1 mole of
produce from 1 mole of

So, 0.0820 moles of
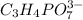 produce from
produce from
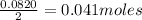 of
of

Now we have to calculate the volume of
 gas.
gas.
Using ideal gas equation:

where,
P = pressure of gas = 1.00 atm
V = volume of gas = ?
T = temperature of gas = 298 K
n = number of moles of gas = 0.041 mole
R = gas constant = 0.0821 L.atm/mole.K
Now put all the given values in the ideal gas equation, we get:
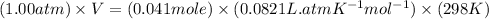
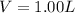
Therefore, the volume of
 gas is 1.00 L
gas is 1.00 L